The Centers for Medicare and Medicaid Services (CMS) has released a summary of each HCPCS Level II code application decision. The document is twenty-eight pages long and the link can be found in the Resources section.
There are 23 items included in the HCPCS Level II Code Q2 2024 Drug and Biological decisions. Twenty new codes have been established. There is one title revision and two code deletions.
| Title | Decision | HCPCS Code | Description |
| Docivyx | Title Revision | J9172 | Change manufacturer from Ingenus to Avyxa |
| Xenoview™ | Add | A9610 | Xenon xe-129 hyperpolarized gas, diagnostic, per study dose |
| Delete | J9150 | Xenon xe-129 hyperpolarized gas, diagnostic, per study dose | |
| Aurlumyn™ | Add | J1749 | Inj, iloprost, 0.1mcg |
| Combogesic ® | Add | J0138 | Inj, acetaminophen 10 mg and ibuprofen 3 mg |
| Paclitaxol Protein Bound Particles | Delete | J9258 | Inj, paclitaxel protein-bound particles (teva) not therapeutically equivalent to J9264, 1 mg. |
| Jubbonti & Wyost | Add | Q5136 | Inj, denosumab-bbdz, biosimilar, 1 mg |
| TYENNE ® | Add | Q5135 | Inj, tocilizumab-aazg, biosimilar, 1 mg |
| TEVIMBRA | Add | Q9329 | Inj, tislelizumab-jsgr, 1 mg |
| KISUNLA™ | Add | J0175 | Inj, donanemab-azbt, 2 mg |
| Baxter’s Vasopressin in Sodium Chloride | Add | J2601 | Inj, vasopressin (baxter), 1 unit |
| Tri-Membrane Wrap | Add | Q4344 | Tri-membrane wrap, per square centimeter |
| Dermacyte® AC Matrix Amniotic Membrane Allograph | Add | Q4343 | Dermatocyte ac matrix amniotic membrane allograph, per square centimeter |
| TheraMend | Add | Q4342 | Theramend, per square centimeter |
| Matrix HD Allograft Dermis | Add | Q4345 | Matrix hd allograft dermis, per square centimeter |
| Artacent C | Add | Q4336 | Artacent c, per square centimeter |
| Artacent Trident | Add | Q4337 | Artacent trident, per square centimeter |
| Artacent Velos | Add | Q4338 | Artacent velos, per square centimeter |
| Artacent VeriClen | Add | Q4339 | Artacent vericlen, per square centimeter |
| AmnioPlast 1 ™ | Add | Q4334 | Amnioplast 1, per square centimeter |
| AmnioPlast 2 ™ | Add | Q4335 | Amnioplast 2, per square centimeter |
| SimpliMax | Add | Q4341 | Simplimax, per square centimeter |
| SimpliGraft | Add | Q4340 | Simpligraft, per square centimeter |
It is recommended to review the application results to determine when the code becomes active or inactive.
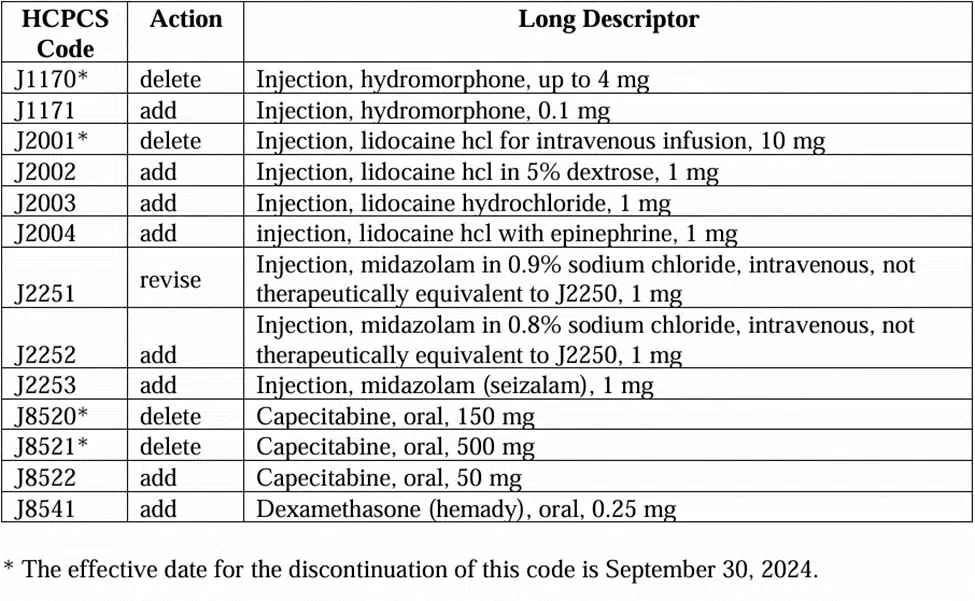
There is also an analysis of HCPCS codes for products approved by the Food and Drug Administration (FDA) under the 505(b)(2) NDA and BLA Pathways and Products Not Otherwise Classified.
The results are eight new codes, one revision, and four deletions as shown below:
The release of the application decisions means that the chargemaster coordinator must review the chargemaster to see if the changes impact the file.
Each of the added items should be discussed with the pharmacy department or chief medical officer (CMO) to determine if there is a plan to add these drugs and biologicals to the formulary. Pharmaceuticals can be difficult because of the translation that is made from the units described in the code description to the usual dosage amount.
The HCPCS codes are updated on a quarterly basis so this process should occur with each release. It is important to avoid unnecessary claim denials due to inactive codes or missed charges because the new codes are not available for reporting.
Resource: https://icd10monitor.medlearn.com/get-ready-for-hcpcs-heres-an-update/