New procedure codes do not impact the MS-DRG assignment.
The Centers for Medicare and Medicaid Services (CMS) released 21 new ICD-10-PCS codes that apply to the vaccination or treatment of COVID-19. These codes are effective January 1, 2021.
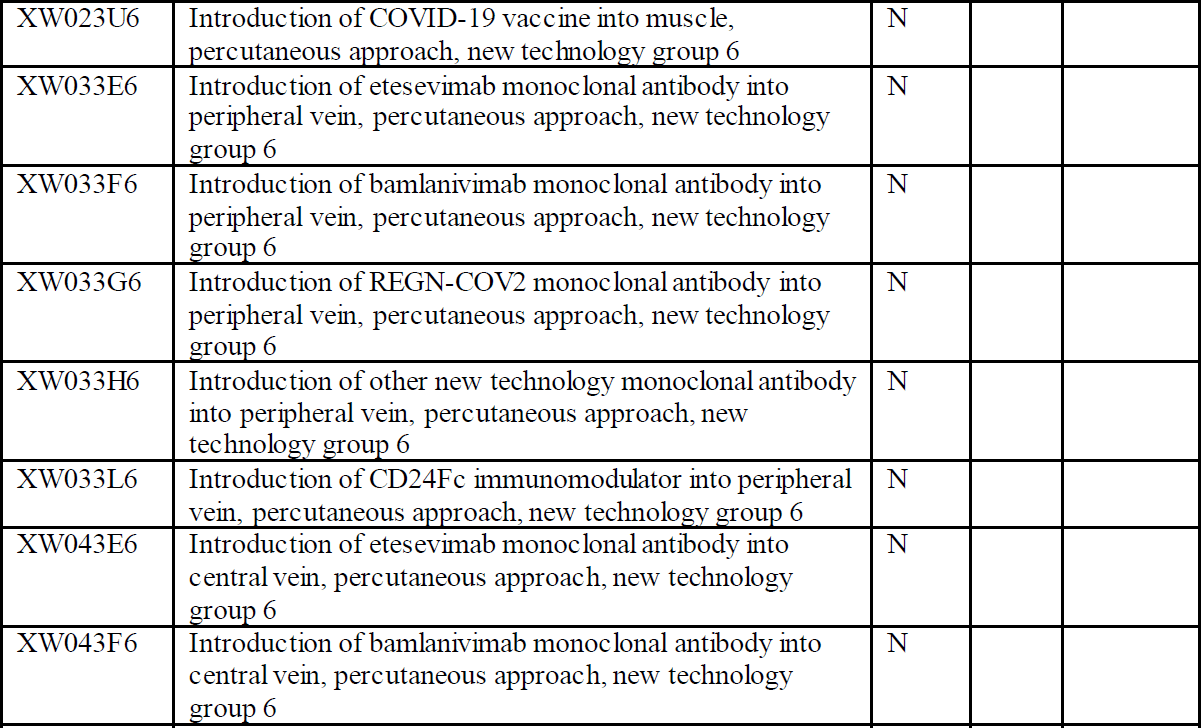
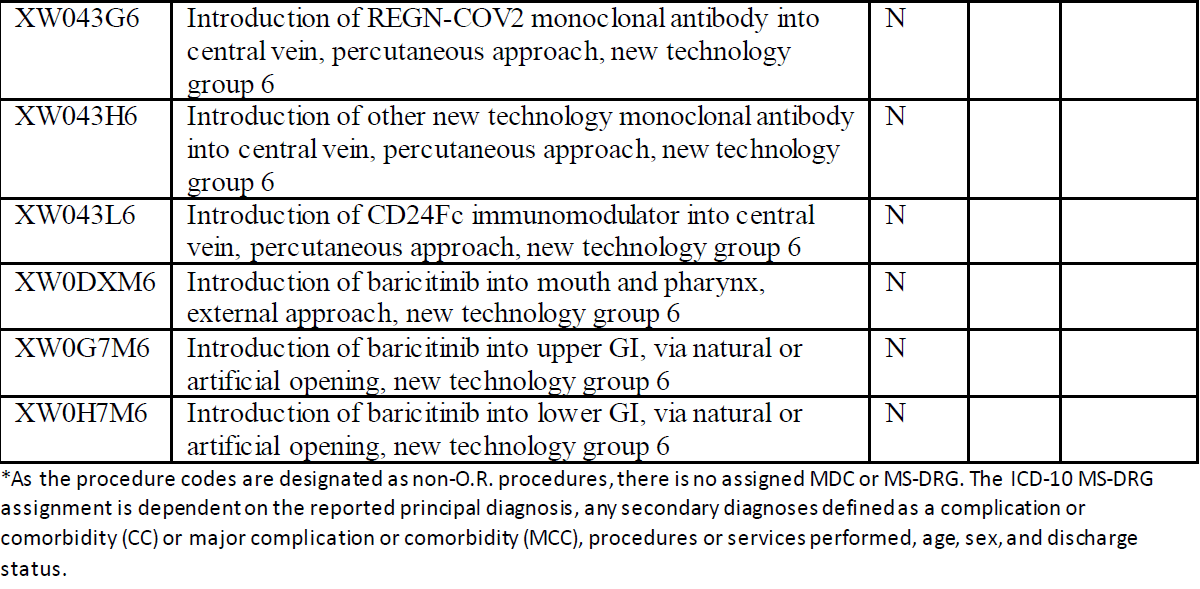
The procedure codes introduce six specific medications that can be used for treatment of COVID-19. The drugs are leronlimab, etesevimab, bamlanivimab, REGN-COV2, CD24Fc immunomodulator, and baricitinib. In addition, an entry for other new technology monoclonal antibody has been added. The approaches vary for the medications so it will be important to review the ICD-10-PCS tables.
New procedure codes do not impact the MS-DRG assignment.
The Centers for Medicare and Medicaid Services (CMS) released 21 new ICD-10-PCS codes that apply to the vaccination or treatment of COVID-19. These codes are effective January 1, 2021.
The procedure codes introduce six specific medications that can be used for treatment of COVID-19. The drugs are leronlimab, etesevimab, bamlanivimab, REGN-COV2, CD24Fc immunomodulator, and baricitinib. In addition, an entry for other new technology monoclonal antibody has been added. The approaches vary for the medications so it will be important to review the ICD-10-PCS tables.
Leronlimab is a monoclonal antibody used to treat HIV. This medication is currently in clinical trials for treatment of COVID. The drug protects health T-cells from viral infection. Etesevimab is a recombinant fully human monoclonal neutralizing antibody which binds specifically to the SARS-CoV2 surface spike protein receptor binding domain. Bamlanivimab is a neutralizing immunoglobulin G1 (IgG1) monoclonal antibody which targets the spike protein of SARS-CoV2. It neutralizes the virus by blocking viral attachment and entry into human cells. REGN-COV2 is an antiviral antibody cocktail which has been tested on rhesus macaques and golden hamsters. CD24Fc (SACCOVID) immunomodulator is a fusion protein which reduces the incidence of viral pneumonia and promotes faster recovery. Baricitinib is a drug that has been approved by the Food and Drug Administration (FDA) for treatment of COVID-19 in combination with remdesivir. This drug combination is used to treat patients who require supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). This drug blocks the activity of one or more of a specific family of enzymes and interferes with the pathway to inflammation.
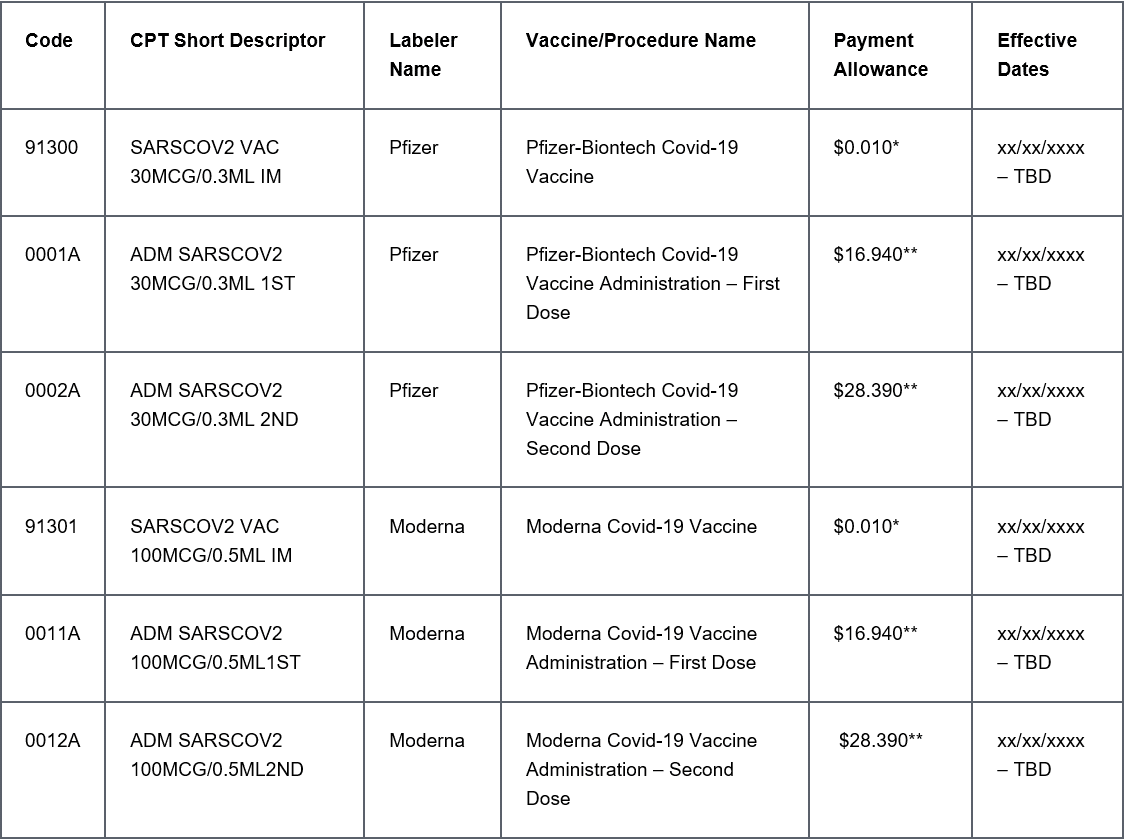
The new codes also include vaccination for COVID-19. The vaccines may require one or two doses of the vaccine. The options include subcutaneous or muscles with a percutaneous approach. These codes do not have the proprietary name of the vaccine included. The new ICD-10-PCS codes are used in conjunction with these CPT® codes:
Payment Allowances and Effective Dates for COVID-19 Vaccines and their Administration during the Public Health Emergency:
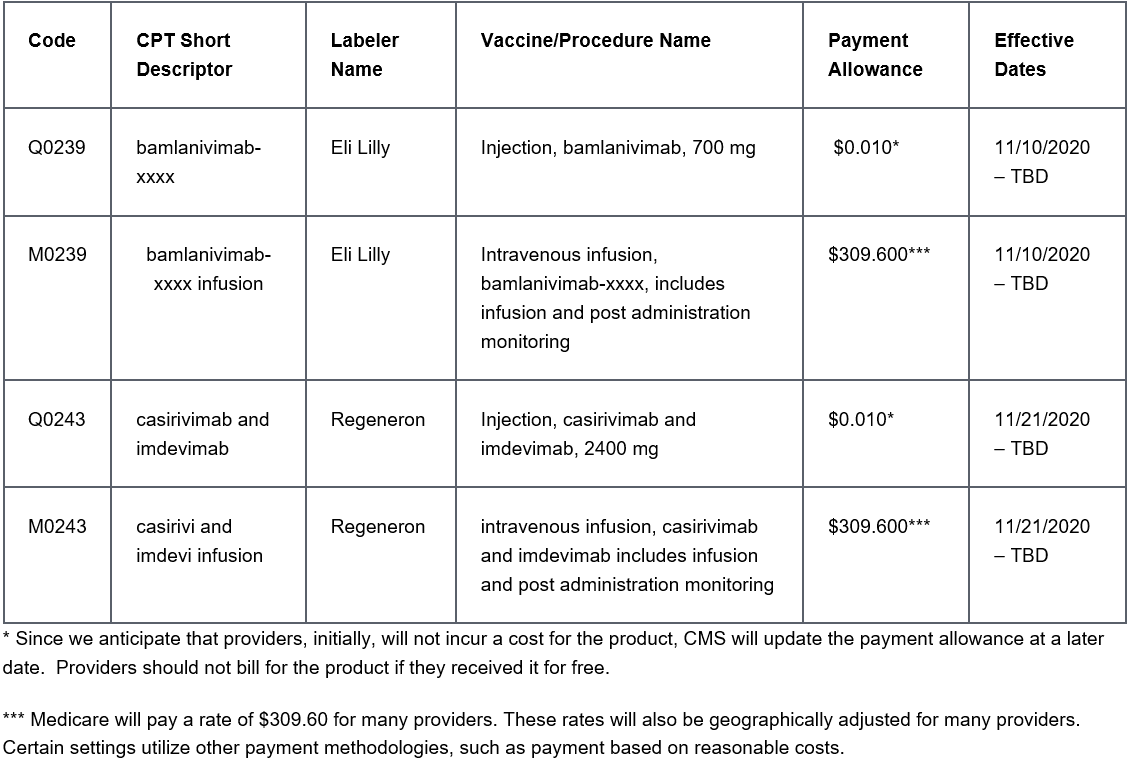
Payment Allowances and Effective Dates for COVID-19 Monoclonal Antibodies and their Administration during the Public Health Emergency:
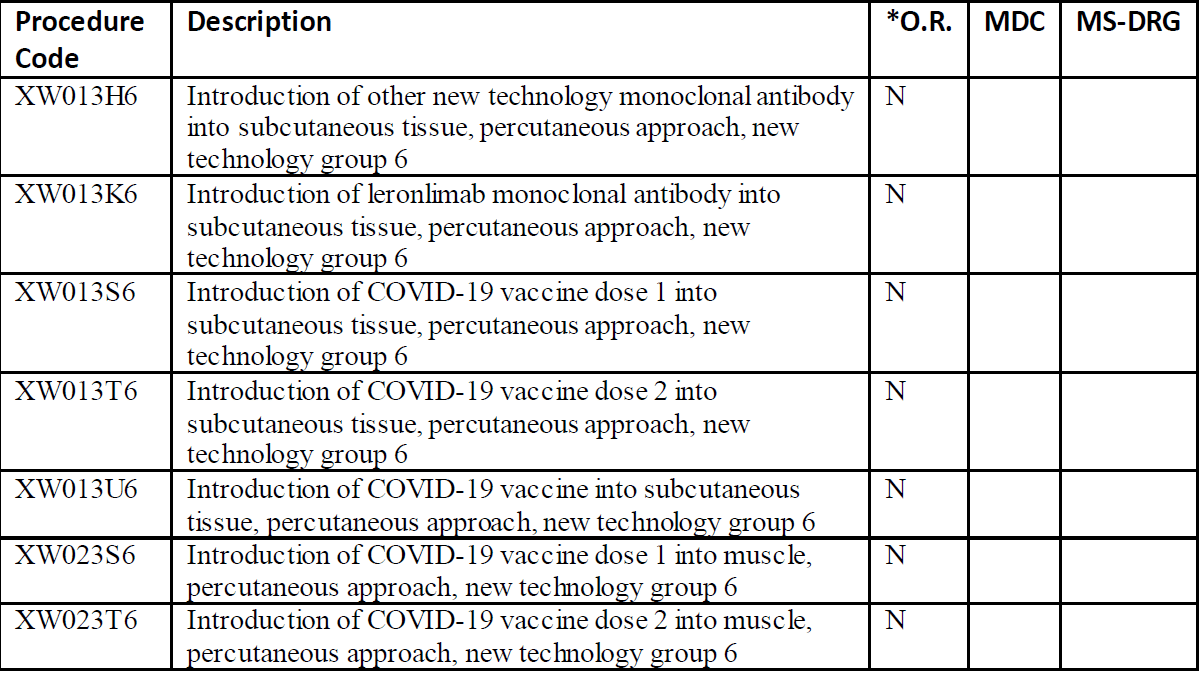
Here is the listing of the new ICD-10-PCS codes per the CMS announcement:
The first decision that should be made is if the inpatient coders will assign these codes. The information can be captured from the detail charges. It is important to review the medications and identify which drugs are used by your facility. Identify the documentation in the electronic health record and communicate this information to the coders. This information can be added to your facility specific guidelines to promote consistent data capture. If you code the vaccination procedures, then the Z23 diagnosis code should be assigned too.
On another note, the Centers for Disease Control and Prevention (CDC) officially listed the new ICD-10-CM diagnosis codes for COVID on December 3, 2020. The addenda have been posted on the CDC website and can be found under Resources. There are six new diagnosis codes that are effective January 1, 2021.
For More Information: https://www.icd10monitor.com/cms-releases-new-icd-10-pcs-codes-for-covid-19